Topics: Math; Programming
Explanation Quality: Better
Medium: Text; Pictures
Type of Content: Educational
 Better Explained is a blog whose motto is "Learn right, not rote." I stumbled across it one day while trying to get a better understanding of the number e. (If you want to know what e is, check out the article on it). My experience with e in school was that it was a number that showed up a lot with exponents and logarithms. No one really explained where it had come from. The article I found on Better Explained really helped me understand. It took the explanation one step at a time, using graphs to approach the idea that e is the base for continuous exponential growth.
Better Explained is a blog whose motto is "Learn right, not rote." I stumbled across it one day while trying to get a better understanding of the number e. (If you want to know what e is, check out the article on it). My experience with e in school was that it was a number that showed up a lot with exponents and logarithms. No one really explained where it had come from. The article I found on Better Explained really helped me understand. It took the explanation one step at a time, using graphs to approach the idea that e is the base for continuous exponential growth.
The thing I like best about this blog is that it helps you get a sort of intuition. The writing style is friendly. The author, Kalid, uses words and pictures as well as math to help you get a sense of what he is explaining.
The blog has about 61 articles talking about math, with the topics ranging in complexity from arithmetic to vector calculus. But all of them are readable, no matter what your level is. There is also a section on programming and web development. I haven't looked at this part much (I do want to learn more about computers, but that's a goal for next summer when I have more time). From what I have seen, the explanations look similar to those in the math section. There are also some articles with tips on doing some computer things like working with Ruby on Rails and debugging.
This blog is worth checking out if you have a specific topic you don't understand, or if you're bored and want to see a new way of looking at something.
Explanation Quality: Better
Medium: Text; Pictures
Type of Content: Educational
The thing I like best about this blog is that it helps you get a sort of intuition. The writing style is friendly. The author, Kalid, uses words and pictures as well as math to help you get a sense of what he is explaining.
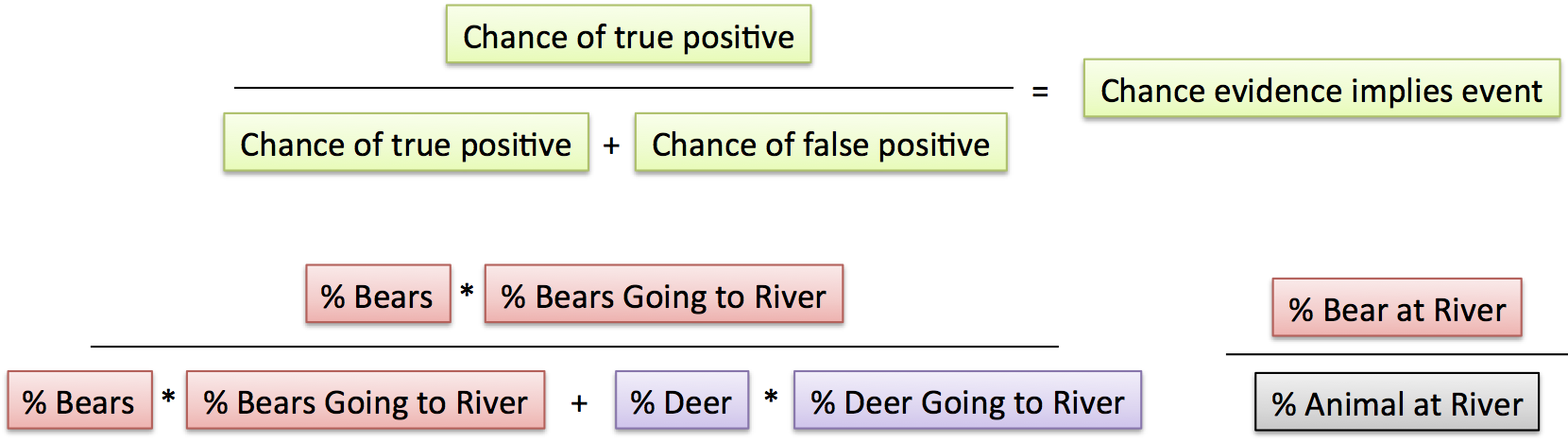 |
| From the BetterExplained article on Bayes Theorem |
The blog has about 61 articles talking about math, with the topics ranging in complexity from arithmetic to vector calculus. But all of them are readable, no matter what your level is. There is also a section on programming and web development. I haven't looked at this part much (I do want to learn more about computers, but that's a goal for next summer when I have more time). From what I have seen, the explanations look similar to those in the math section. There are also some articles with tips on doing some computer things like working with Ruby on Rails and debugging.
This blog is worth checking out if you have a specific topic you don't understand, or if you're bored and want to see a new way of looking at something.


















